Effect of Medicinal Cannabis Therapy (MCT) on Severity of Chronic Low Back Pain, Sciatica and Lumbar Range of Motion
Mustafa Yassin, Avraham Garti and Dror Robinson
DOI10.21767/2471-982X.100014
Department of Orthopedics, Hasharon Hospital, Rabin Medical Center, Petah Tikwa and Sackler School of Medicine, Tel Aviv University, Israel
- *Corresponding Author:
- Dr. Dror Robinson
Head Orthopedic Research Department
Hasharon Hospital, Rabin Medical Center
Keren Kayemet 7, Petah Tikwa, Israel
Tel: 972-3-9372233
Fax: +972-3-9372501
E-mail: dror61@gmail.com
Received date: October 10, 2016; Accepted date: November 21, 2016; Published date: November 29, 2016
Copyright: © 2016 Yassin M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Anecdotal evidence indicates the possible efficacy of cannabis use as an adjunctive treatment in chronic low back pain. The purpose of the current study was to assess the results of treatment of patients suffering from chronic low back pain by medicinal cannabis (MCT).
Methods: A cohort of 46 patients was followed for a minimum of twelve months. They were evaluated at baseline prior to MCT, 3 months later when MCT was begun and up to 12 months of MCT by patient reported outcome questionnaire (SF-12), visual analogue scale (VAS) and the Brief Pain Inventory (BPI), back specific function was assessed using the Oswestry score, range of motion was measured using the Saunders digital inclinometer. Opiate use was assessed using pharmacy dispensation records at baseline and after 12 months of MCT.
Inclusion criteria included: Age over 25 years, sciatica with documented treatment for at least 12 months, evidence on CT or MRI scan of disc herniation or spinal stenosis, failure of at least two narcotic drugs, and consent to use medicinal cannabis. Exclusion criteria included evidence of bone cancer, evidence of diabetic neuropathy, and evidence of prior psychotic reactions.
Treatment protocol: Cannabis usage was at a fixed dosage of 20 grams per month, dose increase was considered at least after 6 months of treatment. The cannabis was smoked at a recommended rate of 4 dosages per day. Results: After 12 months of MCT BPI VAS decreased from 8.4 ± 1.4 to 2.0 ± 2.0; SF12-PCS improved from 47 ± 14 to 55 ± 12; SF12-MCS improved from 44 ± 6 to 50 ± 10; and sagittal plane active range of motion improved from 34º ± 8º degrees to 48º ± 8º,
Conclusion: Short term usage of smoked medicinal cannabis appear to improve both physical and mental function while decreasing pain levels of chronic low back pain sufferers.
Keywords
Low back pain; Cannabis; Spine range of motion; Sciatica; Pain management
Introduction
Medical cannabis use has been gaining momentum recently in some countries [1]. There is still a paucity of knowledge about the use of medical cannabis in chronic low back pain. Low back pain is a common indication for MCT in North America [1]. Preclinical studies have shown that endocannabinoids are involved in low back pain and are affected by certain treatments such as osteopathic manipulation [2]. Data has shown that activation of the cannabinoid CB [1] receptor by synthetic agonists, and pharmacological elevation of endocannabinoid levels, suppress hyperalgesia and allodynia in animal models of neuropathic pain [3]. In fact endocannabinoids were shown to act as anti-allodynia agents through a peripheral [4] and possibly a central mechanism.
The current study was undertaken in order to assess what is the effect of addition of MCT to the treatment of chronic low back pain with a minimal 12 months follow- up of MCT therapy.
Methods
Study design
Due to Ministry of Health regulations the use of MCT is limited to patients who were treated for at least one year by a pain specialist, neurologist or orthopedic surgeon. The treatment should include at least an opiate and one of the atypical analgesics (pregabalin, anticonvulsant\analgesics or anti-depressant). The legal situation dictated a study design which was a cross-over study. The current study is thus an open label, single center, cross-over study of a consecutive series of patients treated by one of the authors (D.R.).
Study temporal sequence
Consecutive patients treated by one of the authors (D.R.) were considered for inclusion in the study during a baseline visit (BL). According to referral letter, medical records, pharmacy dispensation records and patient's history, two points were assessed, i.e. whether the patient has received sufficient therapy and whether the patient had imaging documentation of low back pain accompanied by irradiating symptoms indicative of sciatica. All patients meeting these criteria were administered for 3 months duloxetine 30 mg per day and tramadol 100-300 mg per day for at least three months. Following 3 months of this standardized treatment regimen a second evaluation was performed. Provided the patients were still interested in receiving MCT therapy (MCT 0M), it was begun subsequent to this visit. The MCT dosage was 20 grams per month. No information is available on the exact plant composition, as the patients were randomly assigned to one of 9 available growers. This makes exact determination of THC content impossible. The government regulated system includes coaching of patients regarding the correct way of smoking the substance. MCT was administered by smoking and a 4 times a day 150-175 mg cigarette was recommended. Patients were followed at 6 months (MCT 6M) and 12 months (MCT 12M) after beginning MCT. To reiterate and clarify, the study time-points were as follows: Baseline evaluation -3 months, Beginning MCT – 0 months, 6 months MCT follow-up, 12 months MCT follow-up.
Study inclusion and Exclusion criteria
Inclusion criteria included:
• Age over 25 years due to risk to developing brain of cannabis consumption
• Low back pain and sciatica diagnosed by a study independent orthopedic surgeon with documented sufficient treatment for at least 12 months. Sufficient treatment was determined by the treating physicians who were all studyindependent. The patients were seen in our MCT clinic only after referral by the treating physician due to failure to relieve symptoms for at least 12 months. For all patients, the medical records were checked as well as pharmacy dispensation records in order to make sure that patient were indeed treated and received medication
• Evidence on CT or MRI scan of disc herniation or spinal stenosis
• Treatment for at least 12 months by either a pain clinic, an orthopedic clinic or a neurologist without symptom relief
• Failure of at least two narcotic drugs administered for at least 12 months (cumulative dispensation period)
• Failure of at least one of the atypical analgesics (duloxetine, pregabalin, carbamazepine, amitriptyline, gabapentin, venlafaxine hydrochloride) administered for at least 3 months (cumulative period of dispensation of the pharmaceuticals)
• Signed consent to use medicinal cannabis
Exclusion criteria included:
• Evidence of bone cancer
• Evidence of diabetic neuropathy either per neurologist diagnosis or per electrophysiological studies
• Evidence of prior psychotic reactions (all patients with known psychiatric conditions in the present or the past were evaluated by a psychiatrist to assess risk for drug abuse or psychotic reactions due to MCT, only patients cleared by such consultation were entered into MCT).
Study endpoints
The primary endpoint was the change in pain severity score of the BPI (Brief Pain Inventory) using the two-factor structure originally hypothesized. The BPI has recently been validated for low back pain patients 14. A one grade change in BPI pain severity (10% of total score) was considered as minimal clinically important change.
The secondary endpoints were change in VAS pain intensity during the last week score, change in VAS pain frequency during the last week. These parameters were assessed at baseline as well as after one year therapy. VAS was not assessed at shorter follow-up time periods but only at the annual follow-up as cannabis therapy does take several months to adjust the correct strain per individual. Thus, at 6 months the maximal VAS response is often not yet well defined. For this the VAS score a 30/100 change is considered to be minimal clinically important difference (MCID)7, MCID change in SF12v2 PCS (Short Form 12 version 2 Physical Compound Score) and MCID change in SF12v2 MCS (Short Form 12 version 2 Mental Compound Score) (about 9 points for the two parameters) as well as MCID in the oswestry back disability index in which 10% change was considered MCID as there is no consensus about the correct MCID for this questionnaire but 6.8/100 point was suggested as MCID [5-7].
In addition sagittal range of motion was measured using the Saunders digital inclinometer using factory recommended technique [results are average of three the authors (D.R.)]. The reliability of the measurements is about 5 degrees thus a change of more than 5 degrees was considered as MCID.
Amount of opiate therapy administered was evaluated according to morphine-dose-equivalent (MDE). The dosage was assessed prior to MCT therapy and after one-year, shorter follow-up periods were not evaluated as often the patient has not yet had a chance to visit his pain clinic to change the dosages of opiates due to time required to schedule an appointment.
Statistical analysis
Statistical analysis was performed using the Analyse-It add-in of Microsoft Excel (Analyse-It Software Ltd. 2016, www.analyseit. com). Results are represented as mean ± standard deviation. Significance level was defined at the 0.05 level. Continuous parameters with two time points available were analyzed using the Student's t-test for repeated measures. Continuous parameters with multiple time points were analyzed using oneway ANOVA for repeated measures. Post-hoc analysis using the Bonferroni correction was used in order to define significance of difference against control which was defined as MCT 0M (beginning of MCT therapy). Significance of change in employment status was assessed using the Wilcoxon rank sum test at the 0.05 level.
Results
Demographic characteristics of the cohort
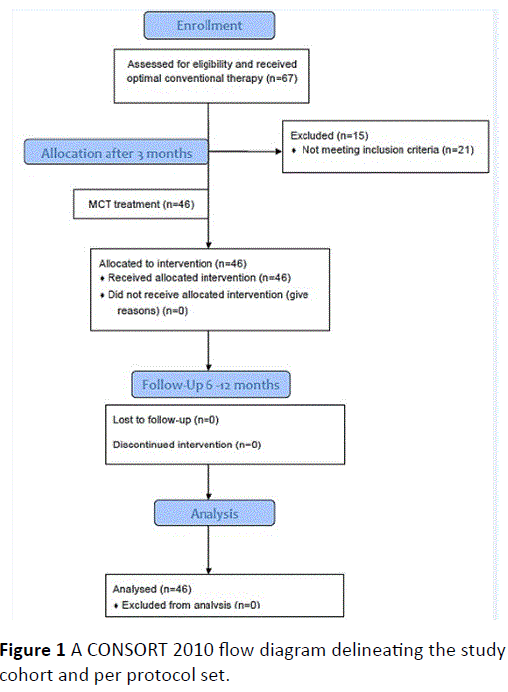
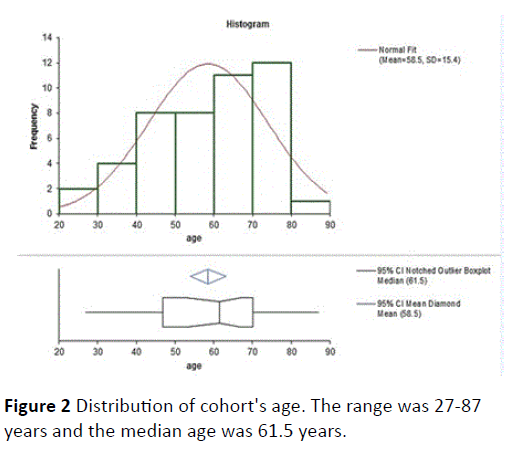
A total cohort of 46 patients fulfilled the criteria (67 patients were screened but some did not meet the rather stringent inclusion criteria regarding 12 months treatment by opiates). A CONSORT 2010 Flow Diagram is shown in Figure 1.
No patients were lost to follow-up and none asked to stop the MCT. Dose adjustment was considered after 6 months based on patient reported shortage of cannabis at the end of the month. The dosage increase was to 30 grams per month. 21 patients required dosage increase due to shortage of cannabis for an average of 10 ± 4 days per month.
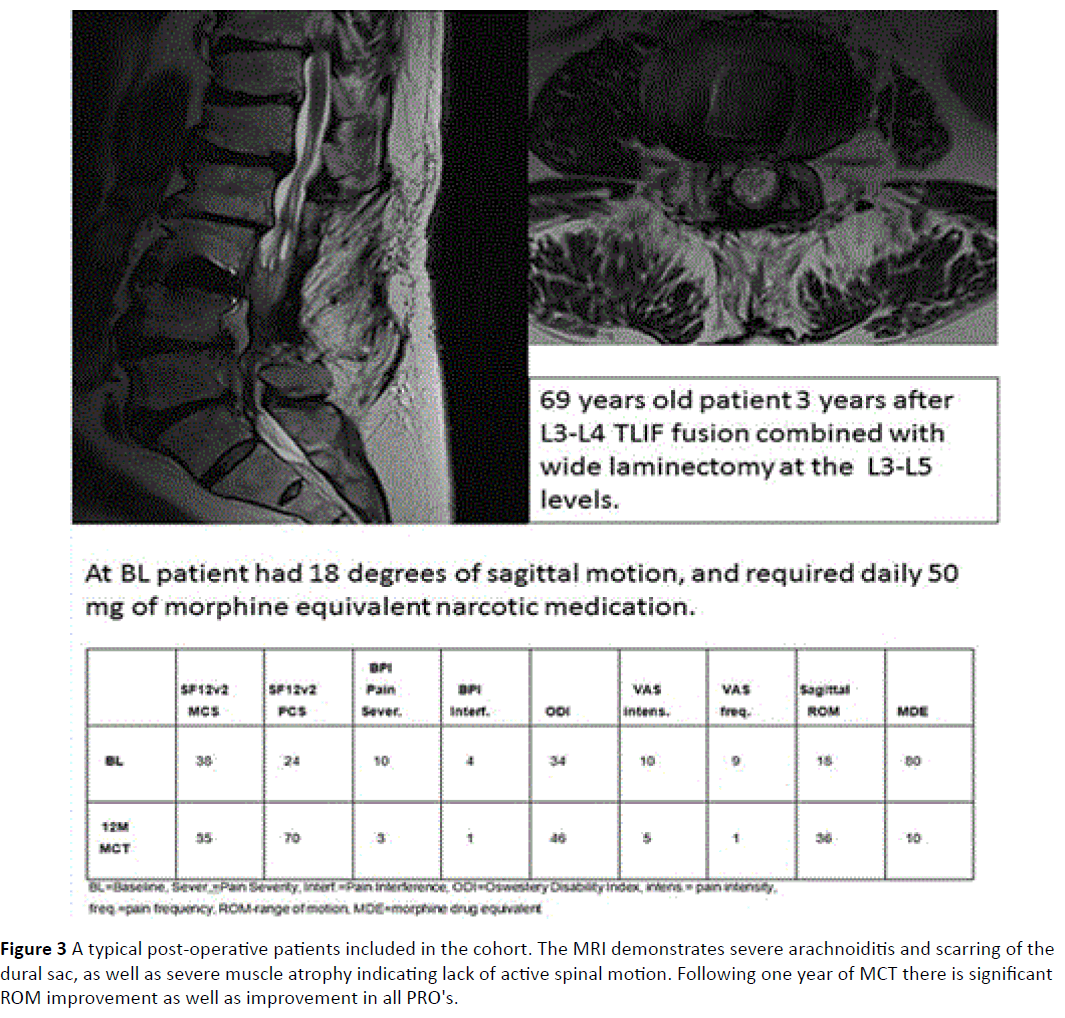
There were 22 males. Average age was 58.5 ± 15.4 years (Figure 2). Nine patients were after fusion surgery with signs of arachnoiditis on MRI (Figure 3). 17 patients had spinal stenosis and 20 had disc herniation or sequestration. Results of PRO's at baseline were similar for all three groups (probably as they are all chronic pain sufferers, and the exact etiology of the pain inciting mechanism is no longer very important symptom determinant). There was no significant change in PRO's results from baseline to MCT 0M except for a change of BPI pain severity but not pain interference between BL and MCT 0M (Bonferroni correction, difference 0.6, 95% confidence interval 0-1.2). Table 1 describes the results of the PRO's, ROM and amount of opiates consumed. A highly significant improvement was noted in all measured PRO's (MCS SF12v2, PCS SF12v2, VAS intensity, VAS frequency, BPI pain severity and BPI pain interference at both post MCT follow-up time-points). The change in opiate consumption was highly significant as well. Change of ROM was significant at the 0.05 level when MCT 0M were compared to MCT 12M results.
| Time Point | SF12v2 MCS |
SF12v2 PCS |
BPI Sever. | BPI Inter. | VAS Intens. | VAS Freq. | MDE | Sagittal ROM | ODI |
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 36.5±8.6 | 33.3±8.2 | 9.2±1.0 | 8.7±1.4 | 9.3±1.3 | 8.7±1.4 | 50.7±33.7 | 35.3±13.7 | 43.7±12.2 |
| MCT 0M | 37.1±8.6 | 37.4±11.2 | 8.6±1.5 | 9.7±1.1 | N/A | N/A | N/A | N/A | N/A |
| MCT 6M | 48.6±7.5 | 54.3±12.6 | 2.7±1.5 | 3.7±1.5 | N/A | N/A | N/A | N/A | N/A |
| MCT 12M | 49.1±8.1 | 55.8±13.0 | 1.1±0.8 | 1.1±0.8 | 3.3±1.7 | 2.0±2.0 | 11.1±21.5 | 41.7±13.0 | 57.8±19.5 |
| Significance | ANOVA p<0.01 |
ANOVA p<0.01 |
ANOVA p<0.01 |
ANOVA p<0.01 |
t-test p<0.001 |
t-test p<0.001 |
t-test p<0.001 |
t-test p<0.05 |
t-test p<0.01 |
| MCT 0M=beginning MCT therapy, MCT 6M=6 months of MCT, MCT 12M=12 months of MCT, MCS: Mental Compound Score; PCS: Physical Compound Score;BPI Sever: BPI Pain Severity Score; BPI Inerfer: BPI Pain Interference Score; VAS Intens: VAS Pain Intensity Grade; VAS Freq: VAS Pain Frequency Grade; MDE: Morphine Drug Equivalent; ROM: Range Of Motion; ODI: Oswestry Disability Index | |||||||||
Table 1 PRO's, Sagittal plane ROM and MDE consumption prior to and during MCT therapy.
Figure 3: A typical post-operative patients included in the cohort. The MRI demonstrates severe arachnoiditis and scarring of the dural sac, as well as severe muscle atrophy indicating lack of active spinal motion. Following one year of MCT there is significant ROM improvement as well as improvement in all PRO's.
A responder analysis is described in Table 2. MCT effect was particularly significant for pain and was practically uniform. Function improvement according to (SF12 compound scores and ODI) occurred in more the half the patients. Only a little short of a third of patients actually had improved ROM. Most patients ROM did not change at MCT 12M compared with MCT 0M (27/46), three patients have actually lost ROM (possibly due to continued scarring of the dural sac as all were post-fusion patients).
| Time Point | SF12v2 MCS |
SF12v2 PCS |
BPI Sever. | BPI Inter. | MDE | Sagittal ROM | ODI | VAS Intens. | VAS Freq. |
|---|---|---|---|---|---|---|---|---|---|
| Number of Responders |
24 | 34 | 46 | 46 | 41 | 14 | 25 | 46 | 46 |
| Responder Percentage |
52% | 74% | 100% | 100% | 89% | 30% | 54% | 100% | 100% |
| Results are represented as delta of result atMCT 12M minus result MCT 0M, responder patient has at least MCID difference in the PRO's.For MDE any reduction of opiate was considered a response. MCS: Mental Compound Score; PCS: Physical Compound Score; BPI Sever: BPI Pain Severity Score;BPI Inerfer: BPI Pain Interference Score;VAS Intens: VAS Pain Intensity Grade;VASFreq: VAS Pain Frequency Grade; MDE: Morphine DrugEquivalent;ROM: Range Of Motion; ODI: OswestryDisabilityIndex |
|||||||||
Table 2 PRO's, Sagittal plane ROM and MDE consumption prior to and during MCT therapy.
MCT safety
No patient in the cohort had to stop the MCT therapy. 19/46 patients complained at MCT 6M visit of red eyes. This was reduced to 6/46 patients at MCT 12M visit. 25/46 patients described increased appetite, which they described as positive experience due to the common gastrointestinal complaints due to opiate treatment. 27/46 patients stopped opiate therapy. 35/46 patients needed less than 20 mg of MDE per day and 42/46 needed less than 30 mg of MDE per day.
No other side-effects of MCT therapy were noted in this cohort of patients. 34 of 46 patients were not yet retired due to age. In this sub-group employment status was recorded. 5/34 patients were working at BL (dropping to 4/34 at MCT 0M) while at MCT 12M 20/34 patients returned to work (difference was significant, Wilcoxon rank sum test, p<0.02).
Discussion
In animals CB1 and CB2 cannabinoid receptors are expressed specifically in hypertrophic chondrocytes of the epiphyseal growth cartilage (EGC) [8], which drives vertebrate growth Cannabis is considered to depress rate of vertebral growth. In a classic model of rat tail disc degeneration, a derivative of cannabis has been shown to delay intervertebral disc degeneration [9]. The authors suggest that cannabidiol significantly attenuated the effects of disc injury induced by the needle puncture. Considering that cannabidiol presents an extremely safe profile and is currently being used clinically, these results suggest that this compound could be useful in the treatment of intervertebral disc degeneration. Can these promising preclinical findings explain the human pain relief and functional improvement observed? Not necessarily. The cohort analyzed consisted of patients suffering from chronic pain for a minimum of one year. There were no differences in MCT response between post-surgical patients, disc patients and spinal stenosis patients. These results might indicate that the effect is more due to pain relieving properties of MCT rather than any structural effects on the human spine. A weakness of this study was the lack of imaging at MCT 12M that might have revealed some structural changes. The authors think that structural changes are unlikely to be affected by MCT therapy. The improvement in ROM in about 30 percent of patients is surprising and might be related to the spasmolytic effect of MCT that is currently used in treating multiple sclerosis patients.
An inherent study weakness is the lack of randomization. Currently such a study is not possible due to regulatory requirements of offering MCT therapy only to patients who failed conventional therapy for at least one year. This requirement also presents a high barrier to success of MCT, as only the worst back pain patients are included in MCT studies. Due to the lack of a control group, it is impossible to rule out the possibility that the improvement was not due to the opiate therapy. This hypothesis does appear unlikely as the patients were administered opiates for one year at least with no improvement. However only a randomized trial can prove this definitely.
Despite this high threshold, MCT appears to be at least in a selected group of patients who failed opiate and atypical analgesic therapy, highly effective. The improvement in life quality is mostly in the physical compound score. The high patient compliance and high rates of return to work, as well as the opiate sparing effect, might indicate that MCT therapy should be considered also in chronic back pain patients, who have not failed opiate therapy for such a prolonged period.
The current results do not let any conclusions be made regarding acute low back pain treatment as the pain mechanisms are probably different than the chronic back pain population treated in this study.
Disclosures
Dr. Robinson and all other authors have nothing to disclose.
References
- Aggarwal SK, Carter GT, Sullivan MD, ZumBrunnen C, Morrill R, et al. (2009) Characteristics of patients with chronic pain accessing treatment with medical cannabis in Washington State. J Opioid Manag 5:257-286.
- Darmani NA, Izzo AA, Degenhardt B, Valenti M, Scaglione G, et al. (2005)Involvement of the cannabimimetic compound, N-palmitoyl-ethanolamine, in inflammatory and neuropathic conditions: review of the available pre-clinical data, and first human studies. Neuropharmacology 48:1154-1163.
- Andreae MH, Carter GM, Shaparin N, Suslov K, Ellis RJ, et al. (2015)Inhaled Cannabis for Chronic Neuropathic Pain: A Meta-analysis of Individual Patient Data. J Pain 16: 1221-1232.
- Comelli F, Bettoni I, Colleoni M, Giagnoni G, Costa B (2009)Beneficial effects of a Cannabis sativa extract treatment on diabetes-induced neuropathy and oxidative stress. Phytother Res 23: 1678-1684.
- Lee JS, Hobden E, Stiell IG, Wells GA (2003)Clinically important change in the visual analog scale after adequate pain control. AcadEmerg Med 10: 1128-1130.
- Parker SL, Mendenhall SK, Shau D, Adogwa O, Cheng JS, et al. (2012)Determination of minimum clinically important difference in pain, disability, and quality of life after extension of fusion for adjacent-segment disease. J Neurosurg Spine 16: 61-67.
- Schwind J, Learman K, O'Halloran B, Showalter C, Cook C(2013) Different minimally important clinical difference (MCID) scores lead to different clinical prediction rules for the Oswestry disability index for the same sample of patients. J Man ManipTher 21: 71-78.
- Wasserman E, Tam J, Mechoulam R, Zimmer A, Maor G, et al. (2015) CB1 cannabinoid receptors mediate endochondral skeletal growth attenuation by Delta9-tetrahydrocannabinol. Ann N Y AcadSci 1335: 110-119.
- Silveira JW, Issy AC, Castania VA, Salmon CE, Nogueira-Barbosa MH, et al. (2014) Protective effects of cannabidiol on lesion-induced intervertebral disc degeneration. PLoS One 9: e113161.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences