Effect of Adding Medical Cannabis Treatment (MCT) to Analgesic Treatment in Patients with Low Back Pain related to Fibromyalgia: An Observational Cross-over Single Center Study
Mustafa Yassin and Dror Robinson
DOI10.21767/2471-982X.100016
Mustafa Yassin and Dror Robinson*
Department of Orthopedics, Hasharon Hospital, Rabin Medical Center affiliated with Tel Aviv University Sackler School of Medicine, Petah Tikwa, Israel
- Corresponding Author:
- Dror Robinson, MD Ph.D
Head Orthopedic Research Unit
Department of Orthopedics, Hasharon Hospital
Keren Kayemet 7 Str., Petah Tikwa, Israel
Tel: +972-39372233
Fax: +972-39372501
E-mail: dror61@protonmail.com
Received Date: July 17, 2017; Accepted Date: August 23, 2017; Published Date: August 30, 2017
Citation: Yassin M, Robinson D (2017) Effect of Adding Medical Cannabis Treatment (MCT) to Analgesic Treatment in Patients with Low Back Pain related to Fibromyalgia: An Observational Cross-over Single Center Study. Int J Anesth Pain Med. 2017, 3:2. doi: 10.21767/2471-982X.100016
Copyright: © 2017 Yassin M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Low Back Pain (LBP) occurs in many patients with fibromyalgia (FM). The current study aimed to assess the possible pain and function amelioration associated with Medical Cannabis Therapy (MCT) in this setting.
Methods: 31 patients were involved in an observational cross-over study. The patients were screened, treated with 3 months of Standardized Analgesic Therapy (SAT): 5 mg of oxycodone hydrochloride equivalent to 4.5 mg oxycodone and 2.5 mg naloxone hydrochloride twice a day and duloxetine 30 mg once a day. Following 3 months of these therapies, the patients could opt for MCT and were treated for a minimum of 6 months. Patient Reported Outcomes (PRO's) included: FIQR, VAS, ODI and SF-12 and lumbar Range of Motion (ROM) was recorded using the modified Schober test.
Results: While SAT led to minor improvement as compared with baseline status, the addition of MCT allowed a significantly higher improvement in all PRO's at 3 months after initiation of MCT and the improvement was maintained at 6 months. ROM improved after 3 months of MCT and continued to improve at 6 months.
Discussion: This observational cross-over study demonstrates an advantage of MCT in FM patients with LBP as compared with SAT. Further studies randomized clinical trials should assess whether these results can be generalized to the FM population at large.
Keywords
Low back pain; Analgesic therapy; Medical cannabis therapy; Fibromylagia
Introduction
Fibromylagia (FM) is a rather common musculoskeletal affliction. A high proportion of patients are affected by Low Back Pain (LBP). This is not surprising as LBP affects 60%-80% of individuals at some point in their lives, and about 10% of cases are related to FM [1]. Treatment of chronic LBP is often multi-modality and not very successful. The results of treatment in patients with LBP in the setting of FM are better in primary FM than in secondary FM [1]. Recently duloxetine has been demonstrated to be effective in chronic musculoskeletal pain conditions [2]. The drug has previously been demonstrated to be effective for pain management in FM and LBP [3].
FM is quite resistant to pharmacologic treatment, and due to its effects on function and patient well-being additional therapies are needed. Medical Cannabis Therapy (MCT) has recently been used in chronic LBP patients with quite remarkable functional improvement [4]. A recent meta-analysis has concluded that most randomized clinical trials of cannabinoids demonstrate that in fifteen of the eighteen trials that met the inclusion criteria demonstrated a significant analgesic effect of cannabinoid as compared with placebo without serious adverse effects [5]. Adverse effects most commonly reported were generally well tolerated, mild to moderate in severity and led to withdrawal from the studies in only a few cases. Previous survey of 1300 patients indicates that MCT is more effective than duloxetine in treating fibromyalgia, with 60 percent of respondents describing duloxetine as ineffective [6]. Endocannabinoid deficiency has been suggested to lead to the ameliorative effect of MCT in fibromyalgia, a disease in which endocannabinoid tone appears dysregulated. All humans possess an underlying endocannabinoid tone that reflects of levels of anandamide. If endocannabinoid function were decreased, it follows that a lowered pain threshold would be operative, along with derangements of digestion, mood, and sleep among the almost universal physiological systems sub served by the endocannabinoid system [7,8].
In order to evaluate the possible role of Medical Cannabis Therapy (MCT) in the management of LBP in FM, a cross-over add-on design of a trial was performed. This design was chosen to increase patients' safety. Thus, no pharmaceutical treatment was stopped but rather the MCT was added on top of current pharmacotherapy.
Methods
Design
Due to national regulations, a treatment trial of 12 months using analgesics is required prior to initiation of MCT for a painrelated indication. To ensure adequacy of analgesic treatment prior to MCT initiation, our clinic mandates a 3 months trial with analgesics prior to consideration of MCT. This policy allows a comparison of the symptom response of the patient cohort treated at our clinic comparing standardized analgesic therapy to MCT. The standardized treatment protocol in our clinic involves duloxetine 30 mg once daily and an opiate delivered twice to thrice daily (most commonly Targin (oxycodone hydrochloride 5 mg and naloxone hydrochloride anhydrous 2.5 mg) as it has less gastrointestinal effects than other opiates.
This study was an observational cross-over trial with participants treated for 3 months by duloxetine and opiates as needed to control their pain. Following a minimum of 3 months therapy, license to treat with MCT was granted by the Israeli ministry of health. The patients could continue the pharmacotherapy used so far and 20 grams of MCT were administered by inhalation of smoke, for 6 months. The study was approved by the hospital ethical committee and registered at ClinicalTrials. gov (NCT03138460). All patients signed a consent form for the cannabis therapy as mandated by national law.
Participants
Participants who met the inclusion criteria were recruited from an orthopedic pain clinic. One of the authors (DR) conducted a thorough physical examination that included neurological testing, postural assessment, joint mobility, strength, etc.
The inclusion criteria were:
1. Symptomatic LBP of more than 12 months duration between T12 and the gluteal fold.
2. History of symptomatic FM for at least 12 months.
3. Failure of opiate therapy of at least 12 months.
Exclusion criteria included:
1. A self-reported history of malignancy
2. Improvement in pain to less than 4 VAS after opiate therapy
3. Refusal to undergo medication by dulexotine and opiates
4. Inability to sign an informed consent form allowing the data to be used for research purposes.
5. Severe cardiovascular disease preventing MCT administration according to a cardiologist
6. Severe psychiatric disease preventing MCT administration according to a psychiatrist
All subjects signed a consent form permitting the use of their data for research purposes, and confidentiality was assured using an anonymous coding system.
Participants were not asked to refrain from receiving other forms of therapy and analgesics during the study.
MCT
MCT therapy is legally obtained from one of several producers following approval by the ministry of health. The approval process requires a minimum period of 12 months of opiate analgesic therapy. All patients considered for treatment of LBP in FM by MCT received a minimum of 12 months' pharmacotherapy from a rheumatologist. As the pharmacotherapy details varied, a period of Standardized Analgesic Therapy (SAT) was administered. Specifically, all patients in the trial received at least 3 months of duloxetine 30 mg once daily and Targin 5/2.5 mg twice a day (each tablet contains 5 mg of oxycodone hydrochloride equivalent to 4.5 mg oxycodone and 2.5 mg naloxone hydrochloride as 2.73 mg of naloxone hydrochloride dehydrate equivalent to 2.25 mg naloxone). This medication is less likely than other opiates to cause constipation and gastrointestinal side effects [9,10]. During this 3 months' period, all other opiates and atypical analgesics were stopped. Following 3 months' treatment patients applied to receive permit for MCT (response time was up to 45 days) and were crossed-over to the MCT arm (however concomitant SAT therapy was allowed). Patients were evaluated prior to standardized analgesic therapy, after 3 months of SAT and after 3 months and 6 months of MCT.
The MCT recommended was 1:4 THC to CBD. The THC levels were less than 5%. The dose of MCT was 20 grams per month for 3 months, administered via smoking or vaporization. After 3 months, according to patient's request and detailed usage table documenting daily the time and amount of drug use, an option to increase the dosage to 30 grams per month was offered.
Outcomes Measures
Outcomes measures included the VAS pain scale to evaluate measure current pain intensity (0 to 10 scale with 10 defined as strongest pain imaginable), Oswestry Disability Index (ODI) to assess functional limitation due to spine disorder (0-100 scale, 0 no disability, 100 total disability) and the revised Fibromyalgia Impact Questionnaire (FIQR) (0-100 scale, 0 total disability, 100 no disability) as well as the seven-rank Patient's Global Impression Of Change (PGIC) Scale (reflects a patient's belief about the efficacy of treatment). PGIC is a 7-point scale depicting a patient's rating of overall improvement. Patients rate their change as “very much improved,” “much improved,” “minimally improved,” “no change,” “minimally worse,” “much worse,” or “very much worse.” [11]. This was reported as numerical values as follows:
1. very much worse
2. much worse
3. minimally worse
4. no change
5. minimally improved
6. much improved
7. very much improved
All these outcome tools are considered valid and reliable quantitative questionnaires to measure the magnitude of clinical change. Physical examination measure that was used for outcome assessment was the modified- modified Schober test to assess lumbar flexion [12,13].
Other endpoints assessed
Analgesic drug use was assessed according to patients' medical records including the list of pharmacy dispensed medications. The use of medication following SAT was graded as 1-need for increased doses, 2-continued dosage required during MCT, 3-decrease by less than one half of medication consumption, 4-decrease by more than one half, 5-no other analgesics consumed.
Study procedure
All participants had SAT drug therapy. Following cross-over SAT could continue and MCT was administered. The patients were examined by one of the authors (DR) every 3 months. Amount of medications dispensed was recorded and the patients filled out the outcome measures. Physical examination was carried out by one of the authors (DR).
Statistical analysis
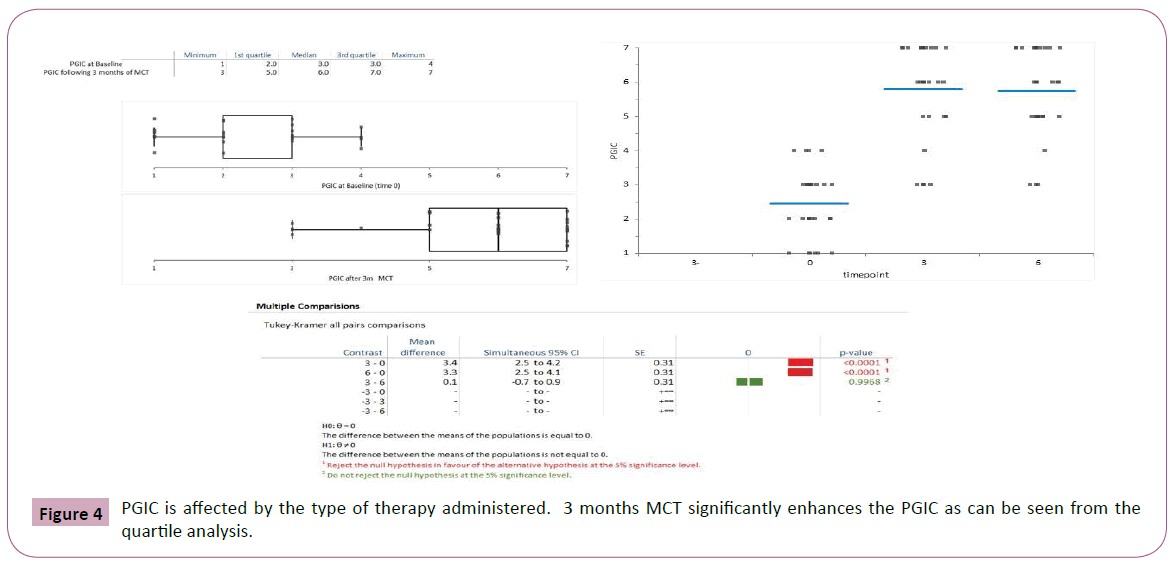
All analyses were performed using the Analyse-it add-on of the for-Microsoft Excel software program. ANOVA was used to compare the outcome scores at various timepoints. After assessing the normal distribution of the data, separate univariate analyses of covariance with time as the covariate were performed to determine whether there were significant differences between the treatment groups scores of functional disability, pain and lumbar ROM. A Tukey-Kramer approach was used to maintain the alpha level at P<0.05. This test compares the means of all pairs of groups.
It controls the error rate simultaneously for all k(k+1)/2 contrasts. Differences with P values ≤ 0.05 were considered statistically significant. Continuous variables were summarized as means and standard deviations.
Results
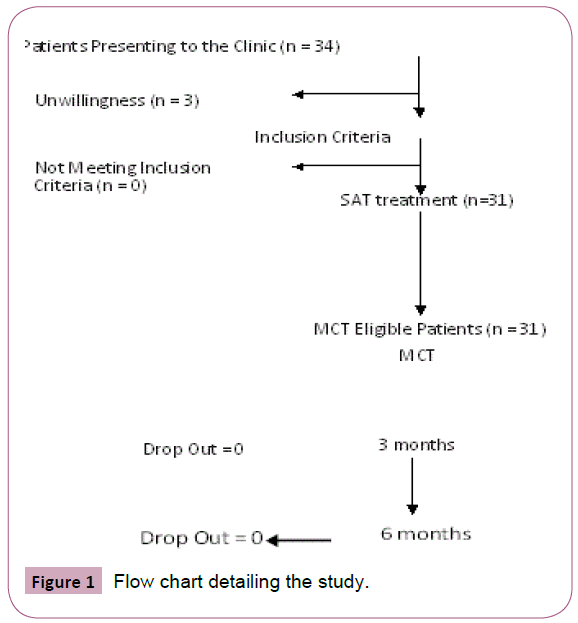
Thirty-one patients with chronic LBP in the setting of FM (28 women, 3 men) aged between 21-75 years participated in the study. Per national regulations, a yearlong therapy of analgesics is required to allow receiving MCT treatment. To make sure that conventional therapy could not alleviate sufficiently their symptom burden, the patients were administered SAT for at least 3 months. 3 patients dropped out and the rest received the MCT arm later. Patient flow through the study is shown in the CONSORT flow chart (Figure 1).
Baseline characteristics of the 31 participants who completed the study are given in Table 1.
| Parameter | n = 31 |
|---|---|
| Mean SD | |
| Age (y) | 33.4±12.3 |
| Onset since first episode (y) | 4.6±3.5 |
| BMI | 29.4±8.5* |
| FIQR score | 46.3±11.1 |
| VAS | 8.1± 1.3 |
| Modified Schober Test (cm) | 3.7± 1.7 |
| Oswestry Disability Index (ODI) | 77.5±10.8 |
*Obesity is very common in our clinic population
Table 1: Baseline Characteristics of Patients with Chronic LBP in FM.
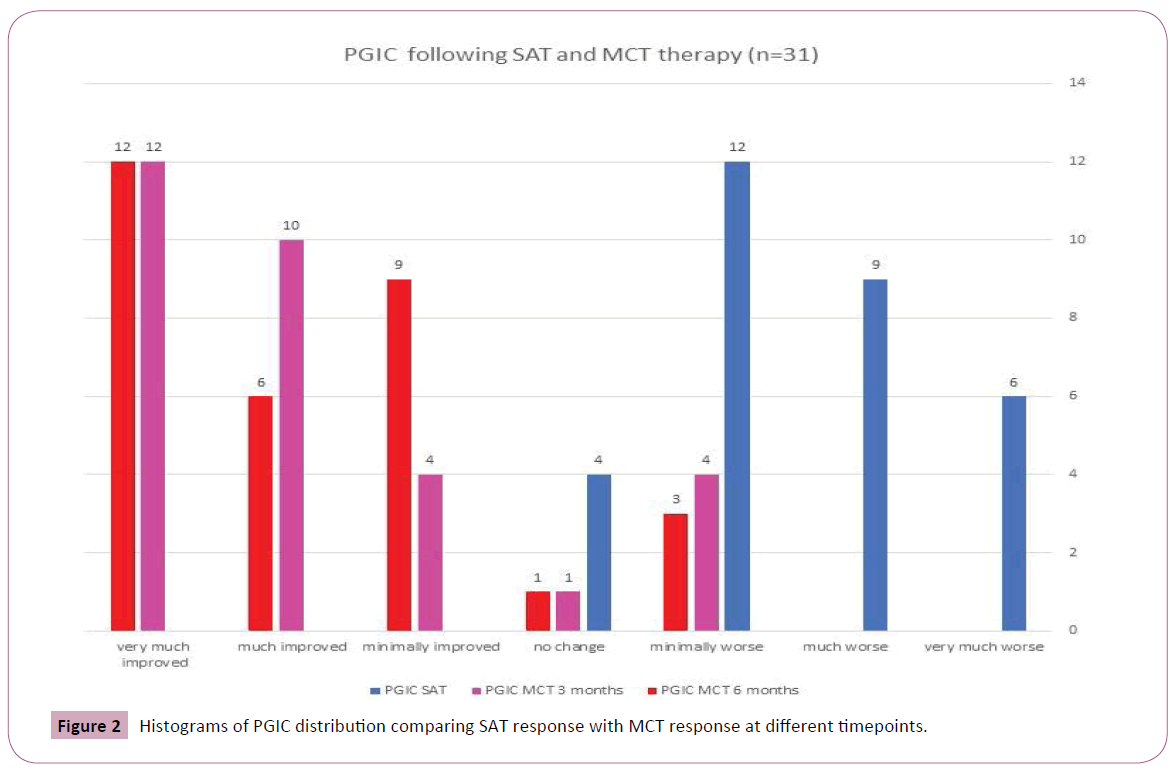
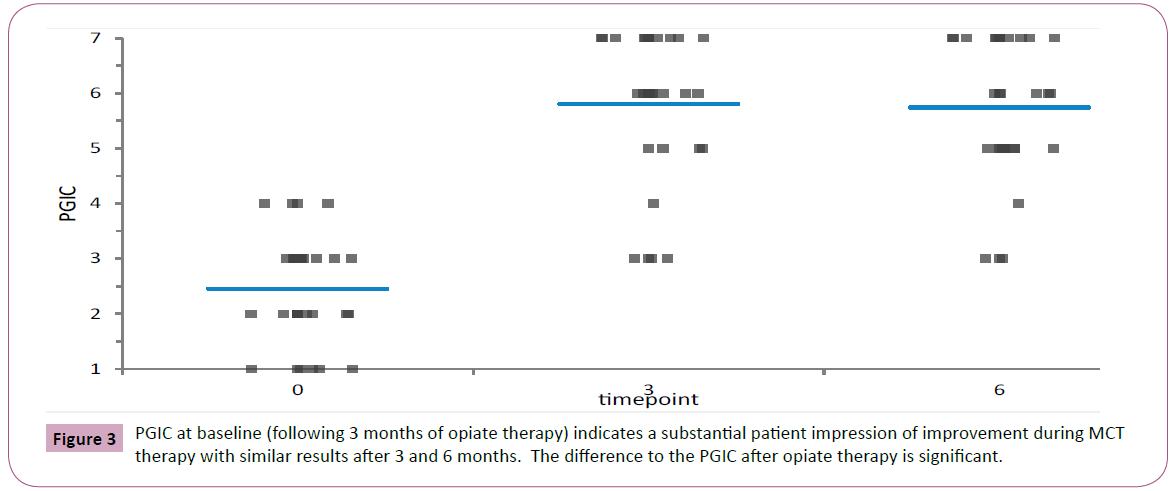
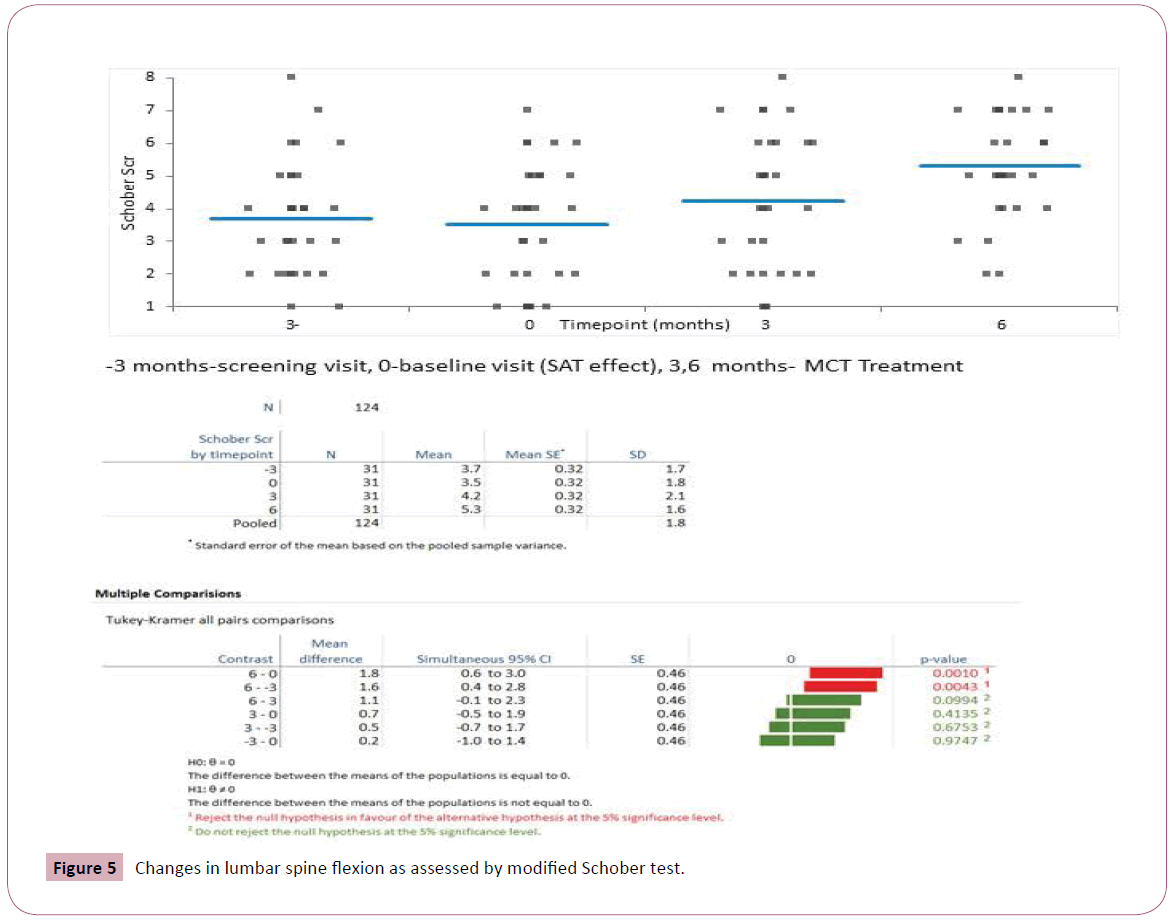
Mean values and standard deviations of the pain intensity, FIQR, ODI and lumbar ROM scores at baseline and at 6 weeks are presented in Table 2. As it can be seen, the opiate treatment allowed minimal improvement in most evaluated functional outcome measures though not in the PGIC (Figure 2). The later addition of MCT allowed a significantly greater decrease of pain concomitant with significant improvement in Schober test. The PGIC was significantly higher in the MCT time interval than in the opiate time interval (Figures 3 and 4, ANOVA p<0.0001).
| Parameter | Screening (-3 months) | Baseline (Time 0) | MCT (3 months) | MCT (6 months) | p Value ANOVA | |
|---|---|---|---|---|---|---|
| VAS | 8.1 ± 1.2 | 8.1 ± 1.4 | 5.3 ± 1.3 | 3.3 ± 2.2 | p<0.0001 | |
| FIQR | 46.3 ± 11 | 45.3 ± 10.2 | 68.7 ± 15.6 | 80.5 ± 12.2 | p<0.0001 | |
| ODI | 77.5 ± 10.6 | 73.7 ± 11.4 | 45.9 ± 19.1 | 30.7 ± 13.6 | p<0.0001 | |
| Schober Test (cm) | 3.7 ± 1.7 | 3.5 ± 1.8 | 4.2 ± 2.2 | 5.3 ± 1.5 | p<0.0001 | |
Table 2 Means and Standard Deviations of Disability, Pain Intensity and Schober test at Various Timepoints.
The spine range of motion was not affected by SAT but improved by 3 months MCT (Tukey-Kramer all pairs comparisons, Figure 4). Interestingly the improvement might continue during therapy with better range of motion after 6 months of MCT though the difference between modified Schober test at 3 months versus 6 months was not significant (p<0.09).
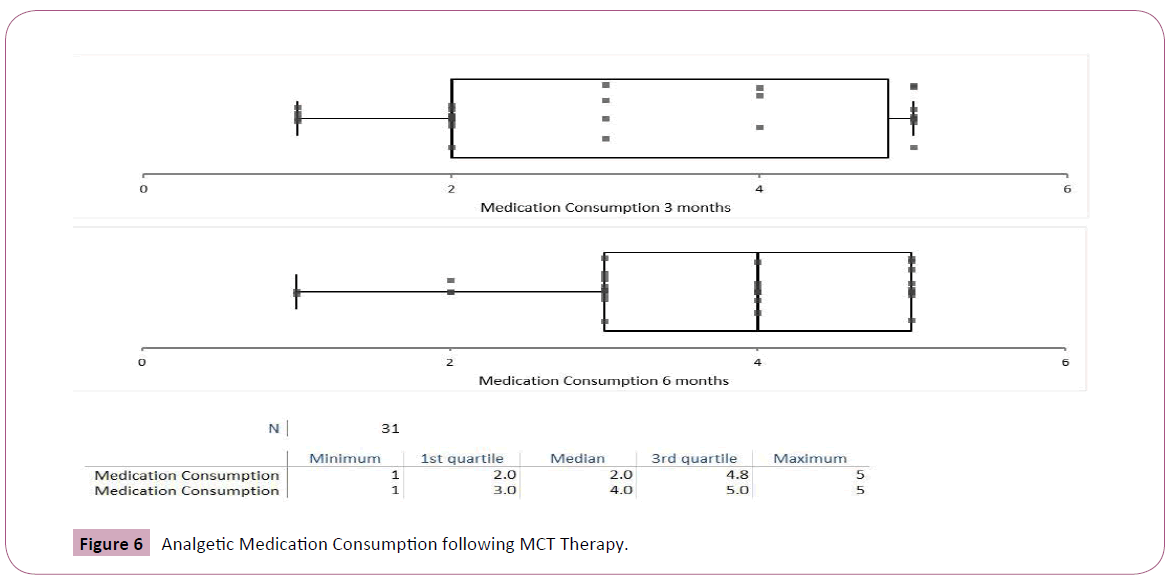
Medications consumption decreased following MCT therapy. The decrease was more marked following 6 months of MCT (Student's t-test 4.5, p<0.001, Figures 5 and 6).
Adverse events during MCT therapy included: red eyes in 28 of 31 patients, increased appetite in 5 of 31 patients, and sore throat in 3 out of 31 patients. The adverse events were mild and did not require MCT alteration. Adverse events during SAT therapy included depression in 2 out of 31 patients, loss of appetite in 8 out of 31 patients, hemorrhoids in 4 out of 31 patients, constipation in 15 out of 31 patients, zombie-like feeling in 5 out of 31 patients. One patient had to be operated for hemorrhoids and in another 6 patients SAT was stopped due to side effects.
Discussion
This study appears to indicate that supplementation of analgesic therapy with MCT alleviate pain in FM patients suffering from LBP. The study design was an observational cross-over study of a consecutive patient cohort. This study design is a requirement of the current national legislation that allows licensing of MCT only to patient who have failed a significant treatment with pharmaceutical analgesics. This SAT procedure might help to select patients who are less likely to have symptoms relief using conventional medication. However, the consort diagram describing the patient flow indicates that most patients who begun SAT did not have pain relief with SAT and went on to MCT. This finding conforms to that of a survey of 1300 patients in which duloxetine and pregabalin were only highly effective in 10 percent of patients with FM and totally ineffective in about 60 percent [6]. The pain relief as well as the PGIC was significantly higher using the MCT therapy. The symptom relief occurs even as early as after 3 months of MCT. The improvement in spinal ROM takes more time and ROM was higher at 6 months as compared with 3 months of MCT.
The mechanism of pain relief by MCT in FM associated LBP is not clear. One option relates to the anti -inflammatory effect of cannabis [14]. Several substances within the inhaled cannabis smoke possess such activity [15]. Increased production of eicosanoids that promote the resolution of inflammation is caused by cannabidiol [15]. Another option is the amelioration of neuropathy by CB-2 receptor activation. A recent meta-analysis has indicated that about 30 percent of LBP have a neuropathic component [16].
A recent Cochrane meta-analysis has concluded that currently, there is insufficient evidence for recommendation for any cannabinoid preparations for symptom management in patients with chronic pain associated with rheumatic diseases. Unfortunately, the included studies did not include any studies using the inhaled whole flower preparation [17]. The effect of synthetic compounds or extracts might differ from that of the whole plant extract both due to different mechanism of entry into the body as well as the possible chaperone effect of the many so called minor molecules present in the whole plant [18]. The improvement observed in range of spinal motion might be due to decreased muscle tonus. Cannabis has been known to reduce muscle spasm and rigidity [19]. In fact, Dr. Russo has suggested back in 2004 that fibromyalgia among other diseases is caused by clinical deficiency of endocannabinoids [20,21]. Subsequent research has supported this concept. The degree of improvement observed following MCT appears higher than that observed using duloxetine in FM [22]. Prior studies employing synthetic cannabinoids have shown that tolerability of FM patients to nabilone is low [23]. The tolerability of MCT in our study was high and no patients had to stop the therapy due to adverse events. The majority of patients elected during MCT to decrease or discontinue pharmaceutical analgesic consumption. Prior research has shown that cannabis has an opiate sparing effect in analgesia [24]. In view of the current opioid dependence epidemic, the usage of treatments that might reduce opiate consumption is of interest.
The disadvantages of the current study include the lack of standardization of the amount of THC\CBD consumed. This deficiency prevents the assessment of a possible dose-response curve in MCT. As FM patients are currently ineligible for MCT therapy, only a small sub-class of FM patients (those with major LBP symptoms) was included. Further research is needed to compare the results of FM patients without LBP to those with LBP. Prior studies have indicated that approximately 40 percent of patients with FM suffer from chronic LBP [25].
The advantages of the current study are that it is the first one to describe results in LBP treatment in the setting of FM. The results appear to indicate improvement in both subjective patient assessed questionnaires as well as spinal range of motion. The decrease in opiate consumption is also an advantage of the MCT therapy. The cross-over design reduces the treatment variability as the results are paired. However, as the patients are aware that limited pain alleviation will allow access to MCT therapy, a potential bias of the SAT arm occurs. This might bias the study results and such bias could only be eliminated by the comparison of a placebo controlled group to the results of MCT therapy. This bias is a cause of concern and a future RCT should be performed.
References
- Borenstein D (1995) Prevalence and treatment outcome of primary and secondary fibromyalgia in patients with spinal pain. Spine (Phila Pa 1976) 20:796-800.
- Brown JP, Boulay LJ (2013) Clinical experience with duloxetine in the management of chronic musculoskeletal pain. A focus on osteoarthritis of the knee. TherAdvMusculoskelet Dis 5:291-304.
- Skljarevski V, Desaiah D, Liu-Seifert H, Zhang Q, Chappell AS, et al. (2010) Efficacy and safety of duloxetine in patients with chronic low back pain. Spine (Phila Pa 1976) 35:E578-585.
- Yassin MGARD (2016) Effect of Medicinal Cannabis Therapy (MCT) on Severity of Chronic Low Back Pain, Sciatica and Lumbar Range of Motion. Int J Anesth Pain Med 2:1-6.
- Lynch ME, Campbell F (2011) Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J ClinPharmacol 72:735-744.
- Anson P (2014) Marijuana Rated Most Effective for Treating Fibromyalgia. National pain report 2014.
- Russo EB (2008) Cannabinoids in the management of difficult to treat pain. TherClin Risk Manag4:245-259.
- Russo EB (2008) Clinical endocannabinoid deficiency (CECD): can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? NeuroEndocrinolLett29:192-200.
- Blaszczyk F, Dron A (2013) Pain therapy with oxycodone/naloxone prolonged-release combination: case report. ContempOncol (Pozn) 17:404-406.
- Morlion B, Clemens KE, Dunlop W (2015) Quality of life and healthcare resource in patients receiving opioids for chronic pain: a review of the place of oxycodone/naloxone. Clin Drug Investig35:1-11.
- Ferguson L. SJ (2009) Patient global impression of change scores within the context of a chronic pain rehabilitation program. Journal of pain 10:73S.
- Tousignant M, PoulinL, Marchand S, Viau A, Place C (2005) The Modified-Modified Schober Test for range of motion assessment of lumbar flexion in patients with low back pain: a study of criterion validity, intra- and inter-rater reliability and minimum metrically detectable change. DisabilRehabil27:553-559.
- Williams R, Binkley J, Bloch R, Goldsmith CH, Minuk T. Reliability of the modified-modified Schober and double inclinometer methods for measuring lumbar flexion and extension. PhysTher 73:33-44.
- La Rana G, Russo R, Campolongo P, Bortolato M, Mangieri RA, et al. (2006) Modulation of neuropathic and inflammatory pain by the endocannabinoid transport inhibitor AM404 [N-(4-hydroxyphenyl)-eicosa-5,8,11,14-tetraenamide]. J PharmacolExpTher317:1365-1371.
- Burstein SH, Zurier RB (2009) Cannabinoids, endocannabinoids, and related analogs in inflammation. AAPS J 11:109-119.
- Fishbain DA, Cole B, Lewis JE, Gao J (2014)What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med 15:4-15.
- Fitzcharles MA, Baerwald C, Ablin J, Hauser W (2016) Efficacy, tolerability and safety of cannabinoids in chronic pain associated with rheumatic diseases (fibromyalgia syndrome, back pain, osteoarthritis, rheumatoid arthritis): A systematic review of randomized controlled trials. Schmerz30:47-61.
- BraidaD, Pozzi M, Cavallini R, Sala M (2001) Conditioned place preference induced by the cannabinoid agonist CP 55,940: interaction with the opioid system. Neuroscience 104:923-926.
- Petro DJ (1980) Marihuana as a therapeutic agent for muscle spasm or spasticity. Psychosomatics 21:81-85.
- Russo EB (2004) Clinical endocannabinoid deficiency (CECD): can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? NeuroEndocrinolLett25:31-39.
- Smith SC, Wagner MS (2014) Clinical endocannabinoid deficiency (CECD) revisited: can this concept explain the therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? NeuroEndocrinolLett35:198-201.
- Skljarevski V, Zhang S, Iyengar S, D'Souza D, Alaka K, et al. (2011) Efficacy of Duloxetine in Patients with Chronic Pain Conditions. Curr Drug ther 6:296-303.
- Walitt B, Klose P, Fitzcharles MA, Phillips T, Hauser W (2016) Cannabinoids for fibromyalgia. Cochrane Database Syst Rev 7:CD011694.
- Lucas P (2012) Cannabis as an adjunct to or substitute for opiates in the treatment of chronic pain. J Psychoactive Drugs 44:125-133.
- Boulanger L, Wu N, Chen SY, Nagar S, Fraser K, et al. Predictors of pain medication selection among patients diagnosed with fibromyalgia. Pain Pract12:266-275.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences