Postoperative Nausea and Vomiting Prophylaxis in Fast-Track Cardiac Anesthesia: A Patient Matched Retrospective before and after Study
Elham Hasheminejad2, Anna Flo Forner1*, Massimiliano Meineri1, Joerg Ender1, Waseem Z A Zakhary1
1Department of Anesthesiology and Intensive Care Medicine, Heart Center Leipzig, Leipzig, Germany
2Department of Anesthesiology and Intensive Care Medicine, Heart Clinic Coswig, Coswig, Germany
- *Corresponding Author:
- Anna Flo Forner
Department of Anesthesiology and Intensive Care Medicine,
Heart Center Leipzig, Leipzig,
Germany,
E-mail: Anna.floforner@helios-gesundheit.de
Received date: August 29, 2022, Manuscript No. IPAPM-22-14455; Editor Assigned date: August 31, 2022, PreQC No. IPAPM-22-14455 (PQ); Reviewed date: September 12, 2022, QC No. IPAPM-22-14455; Revised date: September 22, 2022, Manuscript No. IPAPM-22-14455 (R); Published date: September 29, 2022, DOI: 10.35841/2471-982X.8.5.78
Citation: Hasheminejad E, Flo Forner A, Meineri M, Ender J, Zakhary WZA (2022) Postoperative Nausea and Vomiting Prophylaxis in Fast- Track Cardiac Anesthesia: A Patient Matched Retrospective before and after study. Int J Anesth Pain Med Vol.8 No. 5: 78.
Abstract
In the era of Fast Track (FT) management, Postoperative Nausea and Vomiting (PONV) is a hindrance in achieving the goals of Enhanced Recovery after Cardiac Surgery (ERACS). Aim of our study was to evaluate the efficacy of a Postoperative nausea and vomiting Prophylaxis Protocol (PPP) in FT and to identify independent risk factors for PONV in our study population. This is a retrospective, propensity score-matched, before-and-after study in a tertiary care center. We designed a PPP after a two months quality improvement project on 154 patients to detect PONV incidence in our Post-Anesthesia Care Unit (PACU). To evaluate the efficacy of our protocol we included 262 patients in the study; 131 before and 131 after implementation of PONV prophylaxis. All patients received the same induction and maintenance of anesthesia and were transferred to the PACU to be extubated according to our standard FT protocol.
PONV incidence was reduced from 41.2 to 4.8% (p<0.001) after PPP implementation. History of PONV (OR=4.25), female gender (OR=2.69) and thoracotomy approach (OR=2.48) were identified as independent risk factors. The significant reduction of PONV incidence was observed in all subgroups of patients (history of PONV 80 to 7.7% p<0.001, female gender 65.8 to 7.7% p<0.001 and thoracotomy approach 57.9 to 8.3% p<0.001).
We concluded that our PPP significantly reduced the incidence of PONV in our fast-track protocol in the early postoperative period. This prophylactic regimen could be considered as part of a successful ERACS program.
Keywords
Postoperative nausea and vomiting; PONV prophylaxis; Fast-Track; Cardiac anesthesia; Enhanced recovery
Introduction
Nausea and vomiting continue to be one of the most unpleasant anesthesia-related postoperative complications in several types of surgical procedures including cardiac surgery. The overall incidence of Postoperative Nausea and Vomiting (PONV) after general anesthesia for non-cardiac surgery has been previously reported to be 10-30% and can reach 80% in the presence of risk factors [1,2]. A small number of studies reported an incidence of PONV as high as 45% after cardiac surgery [3]. Beyond subjective patients’ discomfort, PONV may lead to increased risk of electrolyte imbalance, arrhythmias, aspiration, wound dehiscence and gastro-esophageal bleeding [4]. Prophylaxis and management of PONV are very important, especially in the context of Enhanced Recovery after Cardiac Surgery (ERACS) [5,6].
At our institution, we manage many of the elective cardiac surgical patients using a fast track protocol avoiding the Intensive Care Unit (ICU) to enhance postoperative recovery [7].
The aim of this study was to determine the impact of our new prophylaxis protocol in elective cardiac surgical patients managed in a specialized Postoperative Anesthesia Care Unit (PACU) after identifying risk factors for PONV in our patient population. Primary endpoint of our study was to evaluate the rate of reduction of PONV during PACU stay after protocol implementation. Secondary endpoint was to determine independent risk factors for PONV in our study population.
Materials and Methods
After local ethic committee approval (Approval no. 066-15-09032015), we conducted a retrospective observational study in a single university-affiliated heart center. The individual patient consent was waived.
Based on the 2014 PONV prophylaxis guidelines [8], we developed and implemented a PONV Prophylaxis Protocol (PPP) within our fast- track management strategy.
To develop the PONV prophylaxis protocol, we first performed a two-month quality improvement project. Meanwhile the nurses at the bedside were asked to document the occurrence of PONV during PACU stay. We subsequently analyzed the data of 154 consecutive patients to determine the incidence and risk factors of PONV. The risk factors comprised those described in current guidelines [8]: History of PONV and/or motion sickness, non-smoking status, female gender, age, and postoperative opioids. We also assessed additional possible risk factors specific to cardiac surgical patients, such as: arterial hypertension, diabetes mellitus, use of Cardiopulmonary Bypass (CPB), Body Mass Index (BMI), surgical approach (sternotomy vs thoracotomy), intraoperative use of Transesophageal Echocardiography (TEE) and postoperative use of non-invasive ventilation.
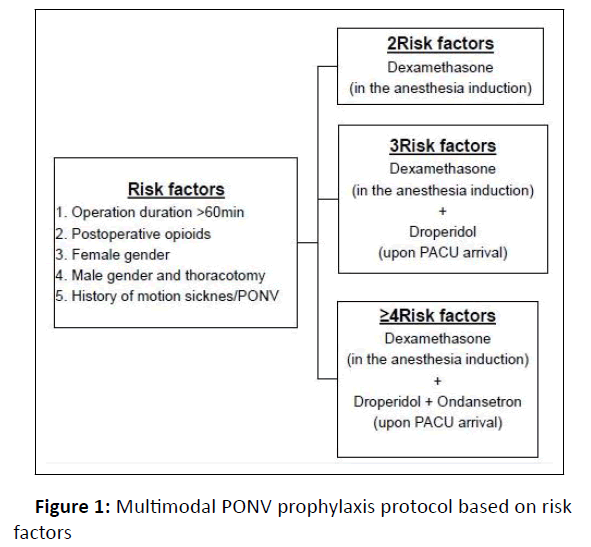
Based on the identified risk factors for our population we designed a specific prophylaxis protocol (Figure 1).
To determine the efficacy of our protocol we then performed a retrospective “before and after” study to compare patients treated before (control group) and those treated after (PPP group) implementation of PPP.
We included consecutive patients older than 18 years who underwent elective cardiac surgery and were suitable for fasttrack management in our PACU in the period from August 2014 to January 2015. Exclusion criteria were: Fast-track failure (defined as an unplanned transfer from the PACU to the ICU) [7] or incomplete documentation. All patients received the same perioperative management.
Patients did not receive any pre-medication. For all patients, anesthesia was induced with Intravenous (IV) fentanyl (200 μg), propofol (1–2 mg/kg) and tracheal intubation was facilitated by injection of a single dose of rocuronium (0.6 mg/kg IV) as neuromuscular blockade. Anesthesia was maintained with a continuous remifentanil infusion (0.2-0.3 mg/kg/min IV) in addition to sevoflurane (0.8–1.1% Minimum Alveolar Concentration). During CPB, the patients received a continuous propofol infusion (3 mg/kg/h IV)
A comprehensive TEE was performed for all surgeries except isolated Coronary Artery Bypass Graft (CABG) with normal left ventricular ejection fraction. A Nasogastric (NG) tube was always inserted after withdrawal of the TEE probe.
At the end of surgery, when fast-track criteria were met and both the surgeon and the anesthesiologist agreed, patients were transferred to the PACU on a continuous infusion of propofol 2 mg/kg/h IV and remifentanil 0.1-0.15 μg/kg/min IV. Once ventilator-weaning criteria were met, remifentanil and propofol infusions were discontinued to allow extubation. All patients were monitored for at least another 2 hours after removal of the endotracheal tube before being transferred to the intermediate care unit [7].
After extubation, pain was assessed hourly using Non-Verbal Rating Scale (NRS) at rest and with coughing during the PACU stay and at PACU discharge.
Piritramide boluses (0.02–0.03 mg/kg IV) were given to maintain a pain score less than 4 NRS as long as the patient did not show signs of excessive sedation (Ramsay score ≥ 3, respiratory rate <10 breaths/min and/or SpO2<95% and/or paCO2>50 mmHg).
Since the integration of PONV prophylaxis in our fast-track protocol in August 2014 all patients received PONV prophylaxis according to the PPP (Figure 1).
To treat PONV in those who did not receive prophylaxis or in case of prophylaxis failure, we chose a different antiemetic drug than that used for prevention (i.e., metoclopramide, dimenhydrinate, or ondansetron) [8].
Sample size, data collection and statistical analysis
We based our sample size calculation on the initial observation of 40% PONV incidence and a desired 50% reduction in the incidence after implementation of protocol [9,10]. Assuming an α of 0.05 and a β of 0.9 we estimated a sample size of at least 118 patients per group as necessary (after Fleiss continuity correction).
We collected the data retrospectively from the clinical information system iMedOne® (Deutsche Telekom Healthcare and Security Solutions GmbH, Germany) and the patient’s chart Medlinq® software (Medlinq, Software-Systeme GmbH Hamburg, Germany) and imported them into standard excel. For data description and analysis we used SPSS (SPSS® Statistics 25.0; Chicago, IL, USA).
Collected data included patients’ demographic data (age, gender, height, weight), past history of PONV and smoking, amount of intra- and postoperative opioids, duration of surgery, cardiopulmonary bypass, aortic cross clamping, as well as the duration of anesthesia, extubation time and length of stay in PACU. We documented the use of PONV prophylaxis and the emergence of PONV in PACU on a binary scale (yes or no).
Continuous variables were assessed for normal distribution using the Shapiro-Wilk-Test. The data are expressed as mean (Standard Deviation (SD)) and compared using student’s t-test when normally distributed, otherwise results are expressed as median (interquartile range). Mann-Whitney-U-Test was used for comparison. Categorical data were expressed as numbers (proportion) and compared using the X2-test or Fisher’s exact test where appropriate.
To determine the risk factors of PONV a multiple logistic regression model was constructed using a backward stepwise selection procedure in which the presence of PONV was the dependent variable. Independent predictors were entered into the model if a significant association (p<0.05) was identified on bivariate analysis and the correlation coefficient between them (co-linearity) was <0.25. Adjusted odds ratios and 95% CIs also were calculated.
In order to minimize selection bias and to obtain comparable groups, a propensity score matching approach was used. For each patient, a logistic regression model was calculated that included variables known to affect PONV and variables that were significant in our bivariate risk factor study. These included: Age, gender, BMI, past history of PONV, smoking status, amount of intra and postoperative opioids, duration of surgery, use of intraoperative TEE and surgical approach. Pairs were matched 1:1 with their nearest neighbor according to the closest propensity score of each subject. Based on the pre-matching range of baseline variable differences, the maximum caliper width for pair-matching was defined at 0.125 of the pooled logit score standard deviation.
Results
One hundred fifty four patients before and 159 patients after PPP implementation were included in our study. Fifty-one patients were excluded after 1:1 propensity score matching, resulting in two equal groups, each consisting of 131 patients. Baseline characteristics and operative data for patients included in the study are not statistically different and are shown in (Table 1).
| Control n=131 |
PPP n=131 |
P | |
|---|---|---|---|
| Age (years) | 67 (57.8-74) | 68 (57.5-76) | 0.509 |
| Gender (female:male) n (%) | 38(29%):93(71%) | 39(29.8%):92(70.2%) | 0.892 |
| Weight (kg) | 82.1 ± 13.8 | 81.2 ± 14.3 | 0.608 |
| Height (cm) | 172.1 ± 9.2 | 171.8 ± 9.8 | 0.788 |
| BMI (kg/cm2) | 27.7 ± 3.8 | 27.5 ± 4.2 | 0.706 |
| aHT n (%) | 105 (80.2%) | 109 (83.2%) | 0.632 |
| DM n (%) | 31 (23.7%) | 37 (28.2%) | 0.481 |
| Ejection fraction n (%) | 60 (50.8-67%) | 60 (55-65%) | 0.648 |
| History of PONV n (%) | 15 (11.5%) | 13 (9.9%) | 0.689 |
| Smoking n (%) | 26 (19.8%) | 16 (12.3%) | 0.129 |
| Surgical approach: | 0.891 | ||
| Sternotomy n (%) | 93 (71%) | 95 (72.5%) | |
| Thoracotomy n (%) | 38 (29%) | 36 (27.5%) | |
| Intraoperative TEE n (%) | 81 (61.8%) | 78 (60%) | 0.8 |
| CPB n (%) | 86 (62-110%) | 82 (50-106%) | 1 |
| CPB time (min) | 63 (40-76) | 59 (30-79) | 0.458 |
| X-Clamp time (min) | 56.4 ± 35.5 | 56.8 ± 39.3 | 0.945 |
| Ventilation Time (min) | 80 (65-111.3) | 80 (55-107.5) | 0.27 |
| NIV n (%) | 53 (40%) | 33 (25%) | 0.064 |
| Piritramid total dose (mg) | 15 (13-21) | 15 (12.5-22) | 0.506 |
| Piritramid (mg/kg) | 0.2 (1.6-2.6) | 0.2 (1.6-2.5) | 0.823 |
| Length of stay in PACU (min) | 265 (220-330) | 255 (215-310) | 0.132 |
Table 1: Demographic characteristics, intra and postoperative data. Values are expressed as mean ± SD, median (25-75% IQR) or number (percentage). Body Mass Index (BMI); Arterial Hypertension (aHT); Diabetes Mellitus (DM); Postoperative Nausea and Vomiting (PONV); Off-Pump Coronary Artery Bypass (OPCAB); Arterial Coronary Bypass (ACB); Minimally Invasive Coronary Artery Bypass (MIDCAB); Transesophageal Echocardiography (TEE); Cardiopulmonary Bypass (CPB); Aorta Cross Clamp (X-Clamp); Non- Invasive Ventilation (NIV); Post Anesthesia Care Unit (PACU).
The overall incidence of PONV before implementation of PPP was 41.2% and there was a statistically significant reduction of PONV after PPP implementation to 4.8% (p<0.001).
The bivariate analysis showed that female gender, history of PONV, thoracotomy and intraoperative use of TEE were significantly associated with PONV. The multivariant analysis proved female gender, history of PONV and thoracotomy to be independent risk factors for PONV (Table 2).
| Bivariate analysis 95% CI for OR | Multivariate analysis 95% CI for OR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk factors | OR | lower | upper | p | B coefficient | OR | lower | upper | p | B coefficient |
| History of PONV | 6.55 | 1.75 | 24.49 | 0.005 | 1.879 | 4.25 | 1.01 | 17.6 | 0.046 | 1.446 |
| Female gender | 3.85 | 1.73 | 8.53 | 0.001 | 1.347 | 2.69 | 1.13 | 6.45 | 0.026 | 0.991 |
| Thoracotomy | 2.39 | 1.11 | 5.15 | 0.027 | 0.87 | 2.48 | 1.09 | 5.6 | 0.029 | 0.908 |
| TEE | 3.22 | 1.49 | 6.94 | 0.003 | 1.17 | |||||
Table 2: Risk factors of PONV. Postoperative Nausea and Vomiting (PONV); Transesophageal Echocardiography (TEE); Confidence Interval (CI); Odds Ratio (OR).
Before PPP implementation the incidence of PONV was significantly higher in women compared to men (65.8% vs. 33.3%, p=0.001), in patients with a history of PONV (83.3 vs 7.1%, p<0.001) and in the thoracotomy compared to sternotomy (57.8 vs 35.8%, p=0.019). In sternotomy subgroup we observed a statistically higher incidence of PONV in women (75% vs 22.2% in women vs men, p<0.001). In thoracotomy subgroup there was no statistically significant difference between genders (50% vs 62% in women vs men, p=0.433).
After PPP implementation the reduction of PONV was statistically significant in all PONV independent risk factors groups: gender, history of PONV and surgical approach (Table 3).
| Control | PPP | P | |
|---|---|---|---|
| Gender | |||
| Male | 31 (33.3%) | 3 (3.3%) | <0.001 |
| Female | 25 (65.8%) | 3 (7.7%) | <0.001 |
| History of PONV | 12 (80%) | 1 (7.7%) | <0.001 |
| Surgical approach | |||
| Sternotomy | 34(36.6%) | 3 (3.2%) | <0.001 |
| Thoracotomy | 22 (57.9%) | 3 (8.3%) | <0.001 |
Table 3: PONV incidence before (control) and after Prophylaxis (PPP) according to independent risk factors
Nevertheless, although the PONV incidence reduction was statistically significant in both genders in patients undergoing sternotomy (from 22.1 to 3% in men and from 76 to 3.7% in women (p<0.001 for both)), for those undergoing thoracotomy, the reduction was only significant in male patients (from 64 to 4.2% in men (p<0.001) vs. 46.2 to 16.7% in women (p=0.202)).
Discussion
Our observational study reported an overall reduction in PONV incidence from 41.2% to 4.8% after implementation of a multimodal PPP as part of ERACS.
PONV has not been extensively studied in the field of cardiac anesthesia, mainly because of the traditional practice of prolonged postoperative sedation and ventilation of patients that did not allow assessment of immediate PONV. A few studies addressing PONV incidence in cardiac anesthesia included various intraoperative anesthesia and postoperative analgesia regimens making the comparison of the results difficult [3,4,11, 12].
The incidence of PONV in our patient population at baseline, before implementation of PPP (41.2%) was comparable to those reported by Grebenik [3], Mace [4], Choi [13] and Champion [14] (nausea 46.5%, 66.5%, 71% and 35.1% respectively and vomiting 36.9%, 34%, 31% and 28.7%).
Early extubation in ERACS may result in increased immediate postoperative pain and need for opioids administration that, among other factors such as intestinal hypoperfusion during CPB may be responsible for the high incidence of PONV in this subgroup of patients [15]. In most cases, PONV is a self-limiting non-serious complication, but it may impede enhanced recovery by delaying the onset of oral intake and mobilization [16].
PONV is multifactorial. Identification of the risk factors to develop risk scores in order to predict the individual probability of PONV and development of target therapy has been largely investigated in general anesthesia [1,2,17,18]. Guidelines for the management of PONV were published in 2014 and updated in 2020 [19]. They aimed at identifying the patients at risk and suggest an evidence-based prevention and treatment regimen consisting of a single or combination therapy [20]. For noncardiac surgery PONV risk factors include: Female gender, age <50, non-smoking status, history of PONV and/or motion sickness, type and duration of surgery >60 minutes, volatile anesthetics, nitrous oxide and postoperative opioids [8].
In our study we confirmed that in cardiac surgery history of PONV, female gender and thoracotomy approach represent independent risk factors. All our cardiac surgical patients underwent >60 min balanced anesthesia and opioid-based postoperative analgesia (which constitute two baseline PONV risk factors). Although volatile anesthetics are a strong risk factor for PONV, their effect depends on the duration of exposure [21]. We routinely substitute sevoflurane with propofol after CPB until the end of surgery and continue it for postoperative sedation, thus not only reducing the exposure to volatile anesthetic but also taking advantage of the anti-emetic effect of propofol. We therefore did not consider the use of sevoflurane as a risk factor [22].
Enhanced recovery programs suggest the use of minimally invasive surgical approaches with the objective to reduce postoperative opioid requirements and to improve recovery [23]. ERACS guidelines do not specifically address either the issue of minimally invasive surgery or PONV prophylaxis in the field of cardiac surgery [24]. Although anterolateral thoracotomy is commonly used in cardiac surgery with very good results, reduction of pain and opioid requirements has not been yet reported. We could not find a clear explanation for the high association of PONV in our male patients undergoing thoracotomy.
Performance of TEE was associated with a higher incidence of PONV but was not identified as an independent risk factor. Stimulation of the upper gastrointestinal mucosal afferent nerves by the TEE probe may delay gastric emptying, but insertion of NG tube for all patients who received TEE should avoid it [25].
The use of prophylaxis protocols in general anesthesia adjusted to the risk scores have been separately reported to be cost-effective for moderate to high risk (≥ 40%) patients (two or more Apfel risk factors) [26] and for all patients with none or one or more risk factors [27]. It has also been demonstrated that different anti-emetics being most widely used such as ondansetron, dexamethason and droperidol show similar efficacy. The use of any of the prophylactic drugs alone reduces PONV by 25% and the use of a combination of drugs has an additive effect. Therefore, the higher the risk of PONV, the more beneficial is the use of a combination therapy to effectively reduce its incidence. Given the lack of evidence for a single antiemetic being more efficient than others, we designed the prophylactic plan based on each patient‘s PONV risk scores and chose prophylactic drugs considering their costs. We designed a PPP based on the recommendations of the PONV guidelines [8] and adjusted to the specific risk factors of our ERACS patients. We chose dexamethasone as first line prophylaxis to be given to all of our patients. The second line prevention included droperidol 1.25 mg IV at arrival to PACU for those patients with an additional risk factor. Droperidol is reported to be the most cost-effective anti-emetic drug at low doses from 0.625 to 1.25 mg IV. It has been shown to be effective in prevention of PONV without increasing the risk of side effects especially the most feared QT-interval prolongation [28,29]. Ondasetron 4 mg IV was administered in PACU as the third line prophylaxis for patients with more than 4 risk factors.
Champion et al. [14] also reported on the implementation of a multimodal anti-emetic regimen to prevent PONV after cardiac surgery. For patients with two risk factors, they used betamethasone 4mg IV postoperatively and for three or more, droperidol 0.625 mg IV was added resulting in a non-significant reduction of PONV (45.5% vs. 54%, p=0.063) in high-risk patients. In comparison to our result (42% vs. 4.8%, p<0.001) they achieved a markedly lower reduction in PONV incidence. This can be partly explained by the fact that they did not include the duration of surgery and postoperative opioids as risk factors, hence undertreated their patients in the active group. They also administered bethametasone upon arrival to the ICU and not at the induction of anesthesia, which may explain a reduced efficacy.
Grebenik et al. [3] added droperidol to the continuous infusion of morphine for postoperative analgesia after cardiac surgery and observed a significant reduction in the incidence of nausea (from 46.5 to 23%) and vomiting (from 36.9 to 22%) without prolonging the extubation time or postoperative length of intensive care. In contrast to our study they used single therapy regardless of the number of risk factors present and therefore they achieved a lower rate of decrease in incidence compared to us. Choi et al. [13] in a prospective randomized study compared the effect of adding placebo, ondansetron or ramsetron to a fentanyl based PCA on PONV incidence and showed a reduction of PONV from 71% (placebo) to 46%(p<0.001) in ondansetron group and 35% (p=0.001) in ramsetron group. They also used single medication for prophylaxis.
Other authors proposed using midazolam as a continuous infusion until extubation to have anti-emetic properties, although it is not recommended in the guidelines. Kogan et al. [9,12] showed a very low incidence of nausea (19.7%) and vomiting (4.3%) when midazolam infusion was maintained during anesthesia and until extubation (8-10hrs postoperatively). Sanjay et al. showed that continuous infusion of midazolam is more effective than ondansetron in preventing PONV after cardiac surgery (nausea 6% vs. 21% and vomiting 0%vs. 21% respectively) with an extubation time of 5h. Our emphasis on early extubation of our patients within 2hrs after surgery is in contrast with using benzodiazepines. In addition benzodiazepines are associated with an increased risk of postoperative delirium in elderly patients [30].
Our study has several limitations. First, it is a retrospective single center observational study. Second, we documented PONV as yes or no answer without differentiation between nausea and vomiting. Third, we only documented PONV in PACU and not a ter discharge to the intermediate care unit.
Conclusion
A multimodal prophylaxis protocol based on the guidelines and adapted to the risk factors of the patients signi icantly reduces the incidence of PONV in cardiac surgery patients treated with an FT protocol.
Given the high incidence of PONV and its potential clinical consequences the use of a PPP in ERACS seems to be justi ied and effective.
Acknowledgments
Elham Hasheminejad and Anna Flo Forner equally contributed to this manuscript
Declarations of Interest
None
Funding
None
References
- Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N, et.al. (1999) A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centersl. Anesthesiology, 91: 693-700.
[Crossref], [Google Scholar], [Indexed]
- Watcha MF, White PF (1992) Postoperative nausea and vomiting. Its etiology, treatment, and preventionl. Anesthesiology, 77: 162-184.
[Crossref], [Google Scholar], [Indexed]
- Grebenik CR, Allman C (1996) Nausea and vomiting after cardiac surgeryl. Br J Anaesth, 77: 356-359.
[Crossref], [Google Scholar], [Indexed]
- Mace L (2003) An audit of post-operative nausea and vomiting, following cardiac surgery: scope of the probleml. Nursing in critical care, 8: 187-196.
[Crossref], [Google Scholar], [Indexed]
- Schwartz J, Gan TJ (2020) Management of postoperative nausea and vomiting in the context of an Enhanced Recovery after Surgery programl. Best Pract Res Clin Anaesthesiol, 34: 687-700.
[Crossref], [Google Scholar], [Indexed]
- Williams JB, McConnell G, Allender JE, Woltz P, Kane K, et.al. (2019) One-year results from the first US-based enhanced recovery after cardiac surgery (ERAS Cardiac) programl. J Thorac Cardiovasc Surg, 157: 1881-1888.
[Crossref], [Google Scholar], [Indexed]
- Waseem Z, Lindner J, Sgouropoulou S, Eibel S, Probst S, et.al. (2015) Independent Risk Factors for Fast-Track Failure Using a Predefined Fast-Track Protocol in Preselected Cardiac Surgery Patientsl. J Cardiothorac Vasc Anesth, 29: 1461-1465.
[Crossref], [Google Scholar], [Indexed]
- Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, et. al. (2014) Consensus Guidelines for the Management of Postoperative Nausea and Vomitingl. Anesthesia & Analgesia, 118: 85-113.
[Crossref], [Google Scholar], [Indexed]
- Sanjay OP, Tauro DI (2004) Midazolam: An Effective Antiemetic After Cardiac Surgery. A Clinical Triall. Anesth Analg, 99: 339-343.
[Crossref], [Google Scholar], [Indexed]
- Santos L, Flo A, Soler M, Monica R, Escudero A, et.al. (2011) Implementation of nausea and vomiting protocol in the ultrafast-track cardiac anaesthesia: 1AP2-9. European Journal of Anaesthesiology, 28: P11
- Hijazi EM, Edwan H, Al-Zoubi N, Radaideh H (2018) Incidence of Nausea and Vomiting After Fast-Track Anaesthesia for Heart Surgeryl. Braz J Cardiovasc Surg, 33 :371-375.
[Crossref], [Google Scholar], [Indexed]
- Kogan A, Eidelman LA, Raanani E, Orlov B, Shenkin O, et.al. (2003). Nausea and vomiting after fast-track cardiac anaesthesial. Br J Anaesth, 91: 214-217.
[Crossref], [Google Scholar], [Indexed]
- Choi DK, Chin JH, Lee EH, Lim OB, Chung CH, et.al. (2010) Prophylactic control of post-operative nausea and vomiting using ondansetron and ramosetron after cardiac surgeryl. Acta Anaesthesiol Scand, 54: 962-969.
[Crossref], [Google Scholar], [Indexed]
- Champion S, Zieger L, Hemery C (2018) Prophylaxis of postoperative nausea and vomiting after cardiac surgery in high-risk patients: A randomized controlled studyl. Ann Card Anaesth, 21: 8-14.
[Crossref], [Google Scholar], [Indexed]
- Gan TJ, Mythen MG (1995) Does peroperative gut-mucosa hypoperfusion cause postoperative nausea and vomiting?. Lancet, 345: 1123-1124.
[Crossref], [Google Scholar], [Indexed]
- Melnyk M, Casey RG, Black P, Koupparis AJ (2011) Enhanced recovery after surgery (ERAS) protocols: Time to change practice?. Canadian Urological Association Journal, 5: 342-348.
[Crossref], [Google Scholar], [Indexed]
- Apfel CC, Heidrich FM, Jukar-Rao S, Jalota L, Hornuss C, et.al. (2012) Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth, 109: 742-753.
[Crossref], [Google Scholar], [Indexed]
- Gan TJ (2006) Risk factors for postoperative nausea and vomiting. Anesth Analg, 102: 1884-1898.
[Crossref], [Google Scholar], [Indexed]
- Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, et. al. (2020) Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting. Anesth Analg, 131: 411-448.
[Crossref], [Google Scholar], [Indexed]
- Kovac AL (2018) Updates in the Management of Postoperative Nausea and Vomiting. Adv Anesth, 36: 81-97.
[Crossref], [Google Scholar], [Indexed]
- Apfel CC, Kranke P, Katz MH, Goepfert C, Papenfuss T, et. al. (2002) Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth, 88: 659-668.
[Crossref], [Google Scholar], [Indexed]
- Song D, Whitten CW, White PF, Yu SY, Zarate E, et.al. (1998) Antiemetic activity of propofol after sevoflurane and desflurane anesthesia for outpatient laparoscopic cholecystectomy. Anesthesiology, 89: 838-843.
[Crossref], [Google Scholar], [Indexed]
- Korsik E, Meineri M, Zakhary WZA, Balga I, Jawad K, et.al. (2021) Persistent and acute postoperative pain after cardiac surgery with anterolateral thoracotomy or median sternotomy: A prospective observational study. J Clin Anesth, 77: 110577.
[Crossref], [Google Scholar], [Indexed]
- Engelman DT, Ben AW, Williams JB, Perrault LP, Reddy VS, et.al. (2019) Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA Surg, 154: 755-766.
[Crossref], [Google Scholar], [Indexed]
- Lavi R, Katznelson R, Cheng D, Minkovich L, Klein A, et.al. (2011) The Effect of Nasogastric Tube Application During Cardiac Surgery on Postoperative Nausea and Vomiting-A Randomized Trial. J Cardiothorac Vasc Anesth, 25: 105-109.
[Crossref], [Google Scholar], [Indexed]
- Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, et.al. (2004) A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med, 350: 2441-2451.
[Crossref], [Google Scholar], [Indexed]
- Jokinen J, Smith AF, Roewer N, Eberhart LHJ, Kranke P, et.al. (2012) Management of Postoperative Nausea and Vomiting. Anesthesiol Clin, 30: 481-493.
[Crossref], [Google Scholar], [Indexed]
- Cao X, White PF, Ma H (2017) An update on the management of postoperative nausea and vomiting. J Anesth, 31: 617-626.
[Crossref], [Google Scholar], [Indexed]
- Henzi I, Sonderegger J, Tramer MR (2000) Efficacy, dose-response, and adverse effects of droperidol for prevention of postoperative nausea and vomiting. Can J Anaesth, 47: 537-551.
[Crossref], [Google Scholar], [Indexed]
- Markota M, Rummans TA, Bostwick JM, Lapid MI (2016) Benzodiazepine Use in Older Adults: Dangers, Management, and Alternative Therapies. Mayo Clin Proc, 91: 1632-1639.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences